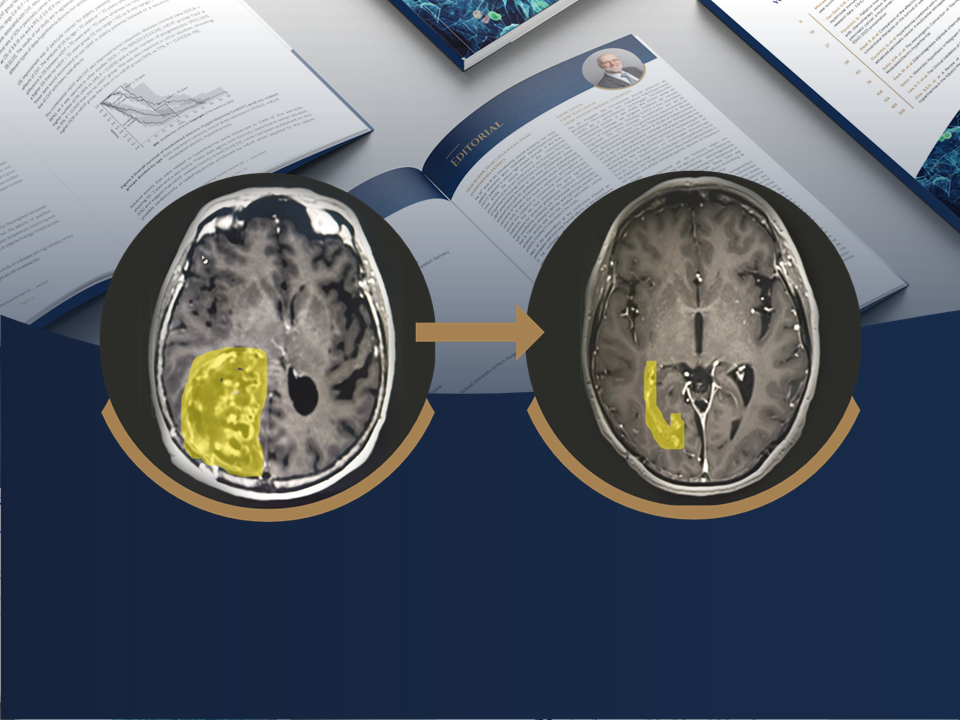
Oncotherm is pleased to announce that its EHY-2030 oncological hyperthermia device has received approval from the Philippines Food and Drug Administration (FDA).
This regulatory clearance confirms that the EHY-2030 meets the applicable safety, quality, and performance requirements, allowing its clinical use in the Philippines.
The approval represents an important milestone in Oncotherm’s international expansion strategy and strengthens the company’s presence in the Southeast Asian medical device market. It also underlines Oncotherm’s long-standing commitment to delivering safe, effective, and innovative oncological solutions worldwide.
This achievement supports our mission to make advanced oncological hyperthermia accessible to a broader range of patients and healthcare providers globally.