MEHT related article in the Cancer of Journal
Óriási szakmai elismerés, hogy az a mEHT-ról szóló publikációnk rákerülhetett a Journal of Cancer borítójára.
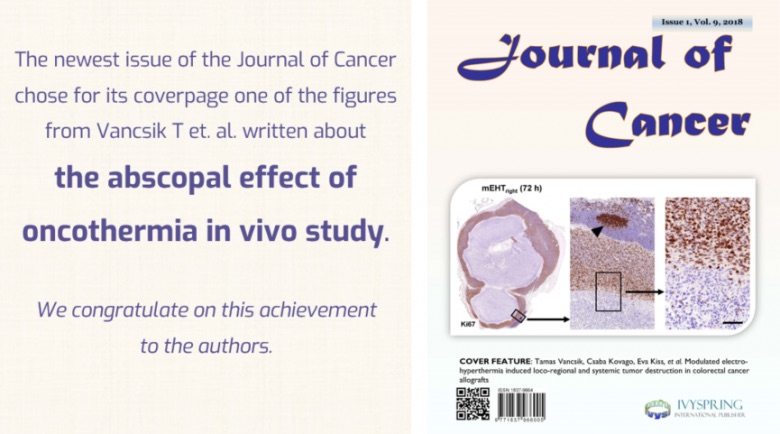
A cikk címe: Modulated electro-hyperthermia induced loco-regional and systemic tumor destruction in colorectal cancer allografts
Ezúton is gratulálunk a szerzőknek!
Szerzők:
Tamas Vancsik,1Csaba Kovago,2Eva Kiss,1Edina Papp,3Gertrud Forika,1Zoltan Benyo,4Nora Meggyeshazi,1,* and Tibor Krenacs1,
Abstract
Background: Modulated electro-hyperthermia (mEHT), a non-invasive intervention using 13.56 MHz radiofrequency, can selectively target cancers due to their elevated glycolysis (Warburg-effect), extracellular ion concentration and conductivity compared to normal tissues. We showed earlier that mEHT alone can provoke apoptosis and damage associated molecular pattern (DAMP) signals in human HT29 colorectal cancer xenografts of immunocompromised mice.
Materials: Here we tested the mEHT induced stress and immune responses in C26 colorectal cancer allografts of immunocompetent (BALB/c) mice between 12-72 h post-treatment. The right side of the symmetrical tumors grown in both femoral regions of mice were treated for 30 minutes, while the left side tumors served for untreated controls.
Results: Loco-regional mEHT treatment induced an ongoing and significant tumor damage with the blockade of cell cycle progression indicated by the loss of nuclear Ki67 protein. Nuclear shrinkage, apoptotic bodies and DNA fragmentation detected using TUNEL assay confirmed apoptosis. Cleaved/activated-caspase-8 and -caspase-3 upregulation along with mitochondrial translocation of bax protein and release of cytochrome-c were consistent with the activation of both the extrinsic and intrinsic caspase-dependent programmed cell death pathways. The prominent release of stress-associated Hsp70, calreticulin and HMGB1 proteins, relevant to DAMP signaling, was accompanied by the significant tumor infiltration by S100 positive antigen presenting dendritic cells and CD3 positive T-cells with only scant FoxP3 positive regulatory T-cells. In addition, mEHT combined with a chlorogenic acid rich T-cell promoting agent induced significant cell death both in the treated and the untreated contralateral tumors indicating a systemic anti-tumor effect.
Conclusions: mEHT induced caspase-dependent programmed cell death and the release of stress associated DAMP proteins in colorectal cancer allografts can provoke major immune cell infiltration. Accumulating antigen presenting dendritic cells and T-cells are likely to contribute to the ongoing tumor destruction by an immunogenic cell death mechanism both locally and through systemic effect at distant tumor sites.